RenovoRX's Latest Innovative Technology Creates New Hope For Chemotherapy Patients

Image Source: Pixabay
Introduction

Wouldn’t it be nice if there was a way to increase the doses of chemotherapy to the tumor whilst at the same time reducing the dose for the rest of the body, as to reduce its horrible side effects?
Well, RenovoRx (RNXT) has actually developed such a technology that it plans to use for solid tumors that are difficult to treat because they have no veins feeding them.
Their patented innovative drug delivery system called RenovoCath uses pressure to deliver chemotherapy agents to reach difficult-to-reach tumors by pressuring them through the walls of a nearby vein.
Doing so enables concentrating chemotherapy in otherwise difficult-to-reach tumors, increasing both the efficacy and lessening the side effects of chemotherapeutic agents.
The company has two clinical trials ongoing, a phase 3 trial for pancreatic cancer and a phase ⅔ trial for duct bile cancer.
RenovoTAMP
Most solid tumors are easily reachable for chemotherapeutic agents as they have plenty of feeding blood vessels going directly into them:
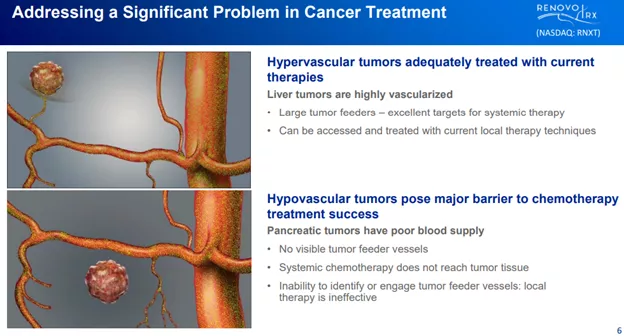
However, there are solid tumors (like pancreatic cancer, duct bile cancer, locally advanced lung cancer, locally advanced uterine tumors, and glioblastoma) that often lack such a direct connection to blood vessels.
This makes these tumors difficult to reach with chemotherapy agents, the alternative is (S-1 registration):
Trans-arterial chemoembolization (TACE) is an established first-line therapy for certain solid tumors. A key component of this approach is to identify and isolate vessels feeding the tumor, known as tumor feeders. However, in patients with pancreatic cancer, no tumor feeder vessels are visible during angiography. In the absence of visible tumor feeders, we can introduce drugs directly across the arterial wall into the surrounding tissue via pressurized diffusion.
Enter RenovoTAMP, their patented drug delivery system, which isolates nearby vessels and uses pressure in these to force the drug into the tumor:
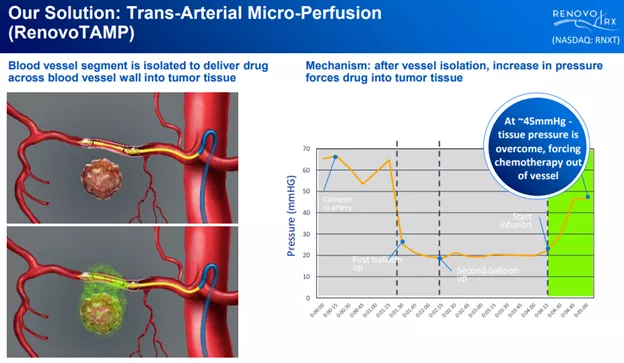
How does that work? Here are the mechanics, from the S-1 registration:
Our RenovoTAMP platform therapy utilizes pressure-mediated delivery of gemcitabine across the arterial wall to bathe the pancreatic tumor tissue in 120mL of saline with 1,000mg/m2 of the drug over a 20-minute delivery time (approximately a total of 1,500-2,000mg of drug dependent upon patient Body Surface Area). RenovoCath is an adjustable double balloon catheter designed to isolate the proximal and distal vessel and adjust the distance between the balloons to exclude any branching blood vessel offshoots.
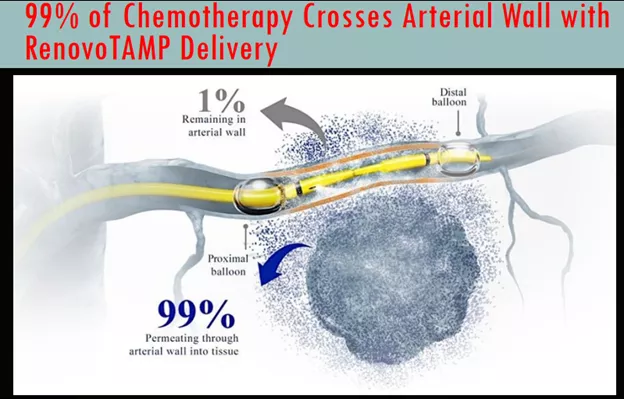
The platform has a number of advantages, from the S-1 registration:
- Application of Approved Small Molecule Chemotherapeutic Agents: We use approved small molecule chemotherapeutic agents such as gemcitabine.
- Targeted Approach: With our approach, we have demonstrated in our clinical studies up to 100 times higher local drug concentration compared to systemic chemotherapy. We believe our approach decreases systemic exposure and improves patient outcomes.
- Delivery Method Independent of Tumor Vascularity: We invented a novel combination platform and delivery system to deliver small molecule chemotherapeutic agents in solid tumors resistant to systemic chemotherapy due to a lack of tumor blood vessels or tumor feeders.
- Broad Application for Solid Tumor Indications: Our platform is not restricted to a single small molecule chemotherapeutic agent or solid tumor type. As such, our platform and delivery system may be applied for use in additional solid tumor indications, including in solid tumors without identifiable tumor feeders.
Pipeline
The company has five conditions in its pipeline, two of which are in the clinical phase:
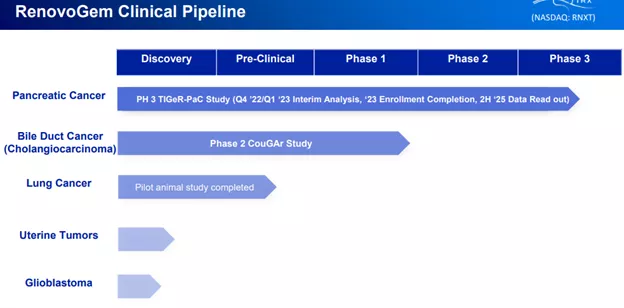
The two candidates in clinical trials for pancreatic and bile duct cancer:
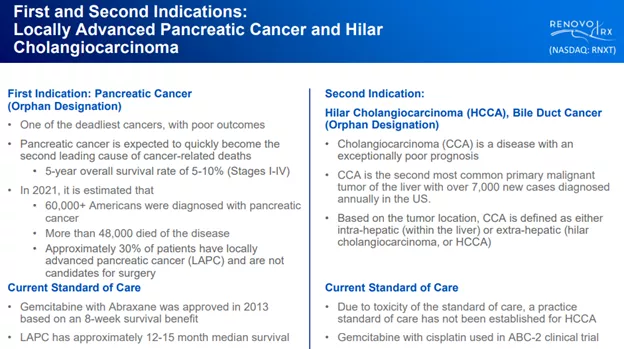
LAPC; Pancreatic cancer with RenovoGem
The most advanced is the company’s treatment for LAPC or locally advanced pancreatic cancer, with RenovoGem, which is a combination of an existing chemotherapy agent gemcitabine delivered via RenovoCath, the company’s patented delivery system.

Mid-previous decade, the company held two clinical trials for RenovoGem:
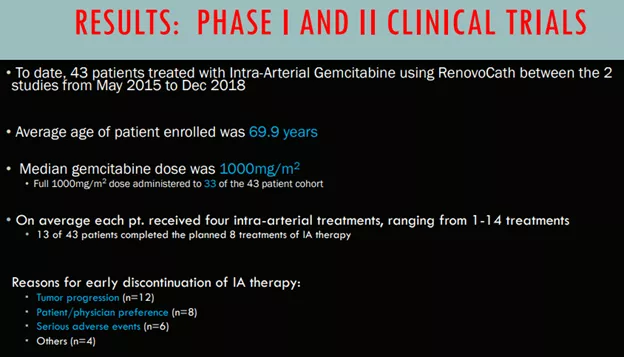
- RR1 a phase ½ safety trial with 20 patients establishing a maximum dose of 1000mg/m2 of intra-arterial gemcitabine delivered via RenovoCath.
- RR2 Observational study produced a 29% survival (versus 12% for chemo) after 2 years.
It has to be said that these were not double-blind studies, the comparison in RR2 is made with an average based on historical data.
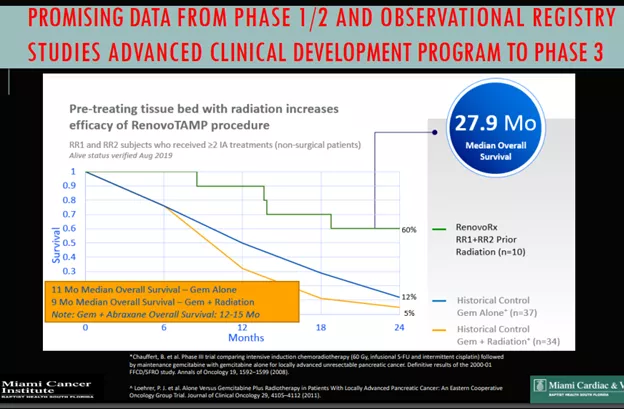
TIGeR-PaC Phase 3 Trial
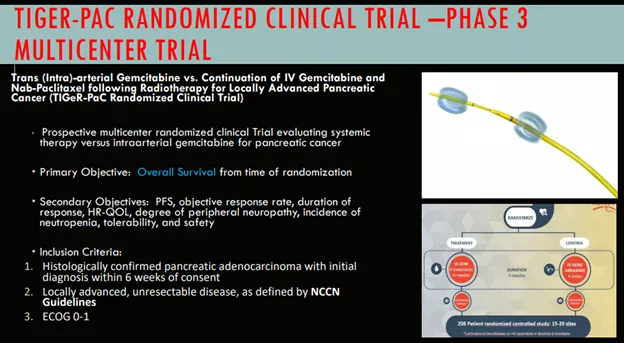
These were encouraging results so they are now continuing with a phase 3 trial called TIGeR-PaC. The phase 3 TIGeR-PaC trial has run into some delays though after the company modified its SAP (statistical analysis plan) which it submitted to the FDA in June 2022. The main changes (from the 10-Q):
- (i) analyze only patients receiving SBRT, consistent with the protocol change made in December 2021,
- (ii) include a second interim analysis,
- (iii) change the total number of SBRT patients randomized in the study to 114 (a reduction from the original200 patients) with a total of 86 deaths from SBRT patients, including all deaths from SBRT patients enrolled in the study before the submission of the Modified SAP, and
- (iv) repower the study from 90% to 80%, which is commonly used in clinical trials.
Originally they were also including IMRT patients but these had a higher dropout rate during the induction phase. In my discussions with RenovoRX CEO Shaun Bagai, he shared that management believes this will shorten the timeframe and significantly reduce costs.

The FDA has not yet signed off on the revised SAP though, in fact, they have not yet submitted the revised SAP, which will occur in Q1/23.
The first interim results will occur when 30% (26 of 86) of the total number of deaths have occurred and the second interim analysis will be at 60% (52 of 86).
On November 14 they had 37 SBRT patients with 114 in total needed, at this rate they expect all patients to be enrolled and randomized in 2025 with the final results out in 2025. But before that happens we get the interim results.
eCCA or extrahepatic cholangiocarcinoma
The company’s second condition for treatment with RenovoGem is eCCA or extrahepatic (or outside the liver) cholangiocarcinoma, cancer that occurs in the bile ducts.
There is already a significant amount of pre-clinical data supporting its effectiveness against this condition.
The company is putting together a phase 2/3 trial treating eCCA with RenovoGem and has already submitted the protocol to the FDA. Without any objection from the FDA, patients can start enrolling in Q1/23.
Market Opportunity

This is for pancreatic cancer alone
RenovoGem gained an ODD or orphan drug designation from the FDA in 2018 for pancreatic cancer and for HCCA (bile duct cancer) in April 2021.
ODD provides the company with seven years of exclusivity to market intra-arterial use of gemcitabine for LAPC and ACCA upon New Drug Application, or NDA, approval.
Finances
The company is not generating revenues and won’t be for quite some time so cash burn is a prime indicator to assess:

There is not a lot of room here as they had $8.1M at the end of Q3 after they pocketed a net $14.6M with their IPO in August 2021. Their GAAP OpEx was $2.1M in Q3 and they lost $7.1M in operational cash flow YTD.
Management expects expenses to increase substantially as a result of the clinical trials, hiring additional research, development, engineering people and SG&A expenses, and defending their IP.
Valuation

There are also 1.2M outstanding performance options, and 2.8M warrants (although with an exercise price of $10.8, they are far out of the money), so that’s 13M shares at $2.7 for a market cap of $35.1M or an EV of $27M.
We’re pretty sure that if the company becomes successful with at least one FDA-approved application for RenovoGem, but keep in mind this is a platform technology with potentially multiple use cases (not to mention it’s not necessarily limited to gemcitabine, the chemical therapy agent they’re using so far).
But we’re not there yet and won’t be for some time, 2025 at the earliest. Before that, they will have to go back to the financial markets and/or find a partner to help them with the clinical trial cost.
Conclusion
RenovoRx takes existing chemotherapies and makes them more effective and less invasive at the same time, by being able to concentrate most of the chemotherapy agent on the tumor itself, rather than letting it loose in the whole body.
All this with the help of its patented RenovoTAMP platform.
This is especially useful for tumors that don’t have feeders, and veins that go directly into the tumor, which is often the case with pancreatic cancer and bile duct cancer, the first two conditions the company is targeting with clinical trials.
Early results are promising, and the company received orphan drug designations for both conditions. But as always this isn’t guaranteed until phase 3 trials are concluded successfully and RenovoGem gets final regulatory approval.
This isn’t imminent, it will be 2025 at the earliest and until then the company will need additional financing and/or a partner with deeper pockets. However, there are two things that make the situation interesting for investors:
- The company’s core technology can be used in multiple conditions, greatly enhancing its potential commercial value.
- The market cap is a fraction of what the company could be worth when they get final FDA approval.
More By This Author:
7 Reasons Why SurgePays Is Going To Surge
Protalix BioTherapeutics Well Placed to Advance in 2023
SOBRSafe: A Very Favorable Risk/Reward Play
Disclosure: This article is part of a new “UnderCovered” series of exclusive articles featuring companies with limited coverage. Authors are compensated by TalkMarkets for their ...
more



I think this news not only bodes very well for the stock price, but for those suffering from tumors as well.
Sounds like promising news.
Very thorough company analysis.
Impressive.
As someone who lost my mom to this, I'm excited to see such a promising advancement in medical therapies. Kudos to #RenovoRX.
Definitely good news for cancer patients.... and $RNXT stock!
Yes, thanks, this would help many people.