Pfizer's Epogen Biosimilar Gets Positive FDA Committee Vote
Pfizer, Inc. PFE announced that an FDA advisory committee has recommended approval of its biosimilar version of Amgen Inc.’s AMGN erythropoiesis-stimulating agent (ESA) Epogen and Procrit.
The Oncologic Drugs Advisory Committee (ODAC) recommended approval across all indications of the reference product, which includes treatment of anemia and chronic kidney disease (CKD).
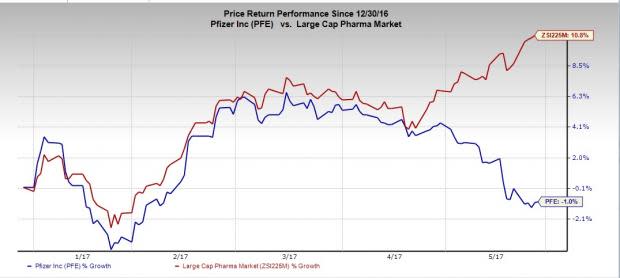
Pfizer’s shares are down 1% so far this year against an increase of 10.8% for the Zacks classified Large-Cap Pharma industry.
This marks the first time a biosimilar version of an ESA has been recommended for approval by an FDA advisory committee. Epogen generated revenues of $1.28 billion in 2016 for Amgen.
We note that Pfizer's biosimilar capabilities were significantly boosted by the Hospira acquisition in 2015. The company launched Inflectra, a biosimilar version of Johnson & Johnson JNJ/Merck & Co., Inc.’s MRK blockbuster drug Remicade in the U.S. in November last year. Inflectra is the first and only biosimilar monoclonal antibody (mAb) therapy and the second biosimilar to be approved in the U.S. Inflectra recorded sales of $17 million in the U.S. and $78 million globally in the first quarter of 2017.
Pfizer’s biosimilars in late-stage development include biosimilar versions of Roche’s Herceptin, Rituxan and Avastin, and AbbVie’s Humira.
Pfizer believes that the market for biosimilars is huge and can grow to $17–$20 billion by 2020. In the first quarter of 2017, Pfizer recorded biosimilars revenues of $105 million.
In fact, the pharmaceutical industry is awaiting a string of biosimilars, which are essentially generic versions of expensive biologic drugs.
Several pharma as well as biotech companies are involved in the development of biosimilars. The first biosimilar to gain approval in the U.S. was Sandoz’s Zarxio, a biosimilar version of Amgen’s Neupogen. Zarxio was launched in the U.S. in Sep 2015.
Other approved biosimilars in the U.S. include Sandoz’s Erelzi, a biosimilar to Amgen’s Enbrel (etanercept). Amgen’s Amjevita is an approved biosimilar version of Abbvie’s Humira. However, these have not been launched yet due to ongoing litigation.
Pfizer carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Ideas with Even Greater Upside?
Today's investment ideas are short-term, directly based on our proven 1 to 3 month indicator. In addition, I invite you to consider our long-term opportunities. These rare trades look to start fast with strong Zacks Ranks, but carry through with double and triple-digit profit potential. Starting now, you can look inside our home run, value, and stocks under $10 portfolios, plus more. Click here for a peek at this private information >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Click for Free Pfizer, Inc. (PFE) Stock Analysis Report >>
Click for Free Johnson & Johnson (JNJ) Stock Analysis Report >>
Click for Free Merck & Company, Inc. (MRK) Stock Analysis Report >>
Click for Free Amgen Inc. (AMGN) Stock Analysis Report >>
To read this article on Zacks.com click here.
Zacks Investment Research
